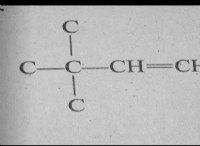
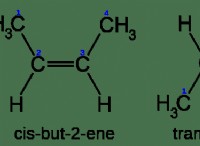
その理由は次のとおりです。
* 物理的変化: Evaporation does not alter the chemical composition of the substance. Water molecules remain H₂O whether they are in liquid or gaseous form.
* 相変化: Evaporation is the transition of a substance from a liquid state to a gaseous state.
* Physical change of state: This further emphasizes that the change is only in the physical arrangement of the molecules, not the chemical bonds themselves.
Other key points:
* Evaporation is an endothermic process 、つまり、周囲からの熱を吸収します。
* Evaporation is driven by the kinetic energy of the molecules 。 Faster moving molecules have enough energy to overcome the intermolecular forces holding them in the liquid state and escape into the gas phase.
Let me know if you'd like to explore any of these aspects in more detail!