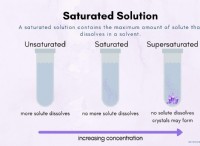
This is because copper sulfate dissociates into copper(II) ions (Cu2+) and sulfate ions (SO42-) when dissolved in water. This means that the compound exists as separate ions in solution, rather than as molecules.
The dissociation of copper sulfate can be represented by the following equation:
CuSO4(s) → Cu2+(aq) + SO42-(aq)
The fact that copper sulfate dissociates into ions in water is evidence that it is an ionic compound.