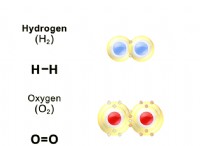
* 原子 :The Law of Conservation of Mass states that matter cannot be created or destroyed in a chemical reaction. これは、方程式の反応物側の各元素の原子の総数が、製品側の各要素の原子の総数に等しくなければならないことを意味します。
* Total Mass :Since atoms are not gained or lost, the total mass of the reactants must equal the total mass of the products. This is a direct consequence of the Law of Conservation of Mass.
* Total Charge :In a chemical reaction, the total electrical charge must remain constant. While atoms may gain or lose electrons, resulting in the formation of ions, the net charge of the system remains balanced.
It's important to note that while these quantities are conserved, the arrangement of atoms changes during a chemical reaction, leading to the formation of new substances.